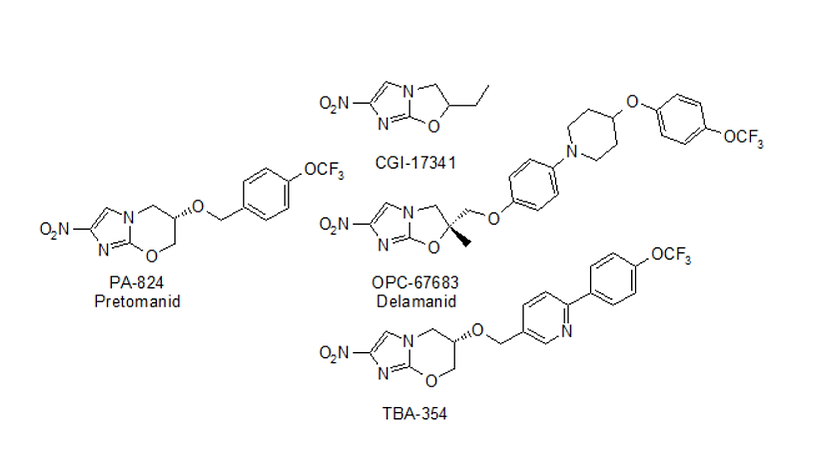
Nitroimidazole Intermediates
Multidrug-resistant and extensively drug-resistant tuberculosis (TB) killed millions of people every year. BPaL and Nitroimidazole drugs Pretomanid, Delamanid’s approving brings hope for our ending TB in the future. Ebuy Chemical Co as a pharmaceutical intermediates Supplier could provide kinds of building blocks.
The US Food and Drug Administration approved a 6-month regimen of pretomanid, bedaquiline, and linezolid (BPaL) for extensively drug-resistant or multidrug-intolerant tuberculosis after a trial in South Africa demonstrated 90% effectiveness 6 months posttreatment.
BPaL was approved by the US Food and Drug Administration (FDA) on August 14, 2019, based in part on results from the Nix-TB trial in South Africa, which included patients with XDR TB or multidrug-resistant (MDR) TB who failed or were intolerant of prior therapy.
The combined activity of BPaL enables cure in a far shorter period compared with currently recommended 18- to 24-month MDR TB regimens. In the Nix-TB trial, BPaL produced favorable outcomes in 98/109 (90%) patients at 6 months posttreatment; in addition, little preexisting resistance to bedaquiline, pretomanid, or line-zolid has been reported.
Pretomanid, the novel agent in the regimen, is a nitroimidazooxazine that blocks cell-wall production in actively replicating MTB organ-isms and acts as a respiratory poison and protein synthesis inhibitor to kill nonreplicating persister organisms.
Nitroimidazole Analogs
Pretomanid was developed over a period of ten years, the recommended dosage, when administered in combination with bedaquiline and linezolid, is 200 mg once daily for 26 weeks; treatment with the combination regimen can be extended beyond 26 weeks if necessary.
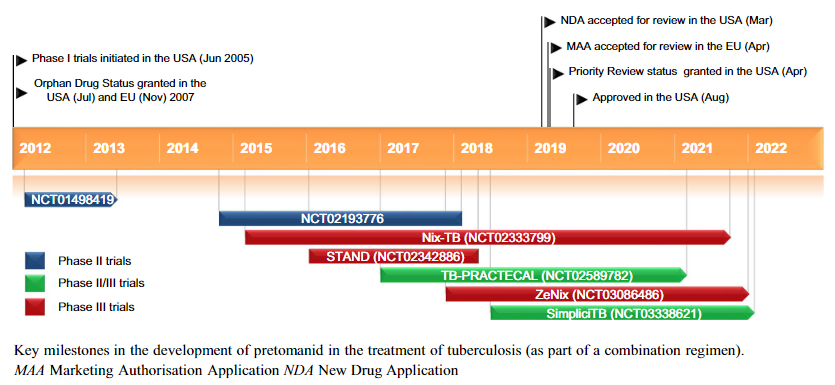
Key milestones in the development of pretomanid
Pretomanid, an oral nitroimidazooxazine antimycobacterial agent administered as part of the BPaL (bedaquiline, pretomanid and linezolid) and BPaMZ (bedaquiline, pretomanid, moxifloxacin and pyrazinamide) regimens, has been developed by the Global Alliance for TB Drug Development (TB Alliance) under license from Novartis, for the treatment for tuberculosis (TB). TB Alliance has licensed Mylan to manufacture and commercialize pretomanid for use as part of the BPaMZ and BPaL regimens. The license is non-exclusive in low- and middle-income countries and exclusive in high-income markets.
Pretomanid pharmacokinetics after oral, single-dose administration were approximately dose proportional over a dose range of 50–200 mg and less than dose proportional over a dose range > 200–1000 mg. Administration of pretomanid with a high-fat, high calorie meal increased expo-sure to pretomanid (mean Cmax increased by 76% and mean AUC ∞ by 88%); the drug should be taken with food. After a single 200 mg dose of pretomanid administered with food to healthy adults, the mean Cmax of 2.0 μg/mL was achieved in a median 5.0 h and the mean AUC ∞ was 53.0 μg h/mL. Pretomanid is ≈ 86.4% bound to plasma protein and the estimated mean apparent volume of distribution is 97 L. Steady state was achieved ≈ 4–6 days after repeated administration of pretomanid 200 mg once daily under fasted conditions in the same population. The accumulation ratio was ≈ 2 under fasted conditions; the steady state mean Cmax (1.7 μg/mL) was achieved in a median 4.5 h and the mean AUC 24 was 30.2 μg.h/mL.
Pretomanid is metabolized by multiple reductive and oxidative pathways, with no single pathway dominating. In vitro studies, CYP3A4 accounted for up to ≈ 20% of pretomanid metabolism. Pretomanid is excreted in urine (53% of a dose) and faeces (38%), predominantly as metabolites ≈ 1% of a dose is excreted in urine as the unchanged drug. The estimated mean apparent oral clearance of pretomanid after a single 200 mg dose under fed conditions in healthy adults is 3.9 h and the estimated mean half-life is 17.4 h. Bodyweight, sex, race, HIV status and pulmonary TB status (XDR, treatment intolerant or non-responsive MDR) has no clinically significant effect on pretomanid pharma-cokine tics. The impact of patient age (≥ 65 vs < 65 years) and renal or hepatic impairment on pretomanid pharmacokinetics is not known. Co-administration of pretomanid with the CYP3A4 inducers efavirenz and rifampicin reduced pretomanid plasma concentrations; consequently, coadministration of BPaL with efavirenz and rifampicin or other moderate or strong CYP3A4 inducers should be avoided.
Pretomanid significantly inhibited the OAT3 drug transporter in vitro, which suggests that concentrations of OAT3 substrate drugs (e.g., methotrexate) could be increased at clinically relevant pretomanid concentrations. If coadministration is required, monitoring for OAT3 substrate drug-related adverse events is recommended and dosage reduction of OAT3 substrate drugs may be required. The features and properties of Pretomanid was summarized in Figure 3.
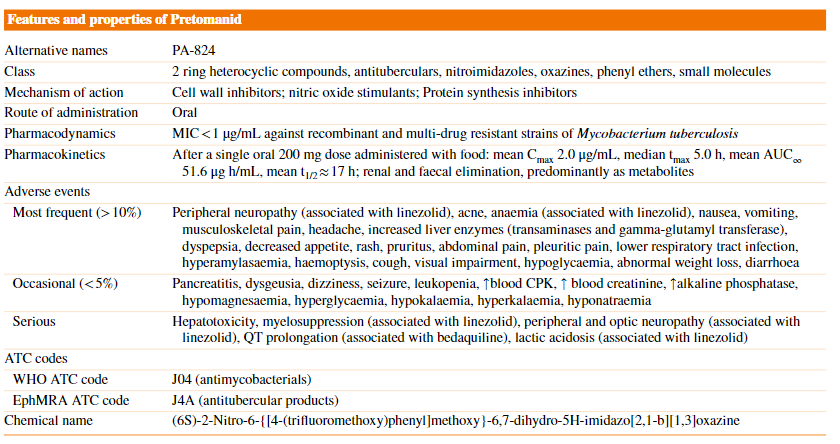
The features and properties of Pretomanid
The synthesis of Pretomanid was summarized in Figure 4, 2-bromo-4-nitro-1H-imidazole (CAS: 65902-59-2) as the starting material, Ebuy Chemical as a pharmaceutical intermediates Supplier could provide this scaffold from gram to kilogram with high quality. Nitroimidazole intermediate such as 2-nitroimidazooxazine and 6-nitroimidazooxazole subclasses could be used for the synthesis of tuberculosis (TB) drug like Pretomanid, Delamanid, TBA-354 and so on.

Kilogram Scale up of pretomanid
Glycidol was needed during the alkylation, Ebuy Chemical could also provide (R)-(+)-Glycidol (CAS: 57044-25-4) and (S)-(-)-Glycidol (CAS: 60456-23-7) as well, protected Glycidol has become an important key intermediate for the preparation of chemicals, pharmaceuticals, and bioactive compounds. It was used for the preparation of compounds containing epoxide functionality. For example, the reaction of glycidol with isocyanates yields glycidyl urethanes as commercially important materials. A part of the reactivity of glycidol depends on the oxiran ring and acts as an alkylating agent. Moreover, it was used in the production of flavoring and sweetening agents. Glycidol is used as a stabilizer in the production of vinyl polymers and natural oils.
Although the glycidol scaffold is found in two commercially important groups of derivatives, glycidyl ethers ((S)-(+)-Benzyl glycidyl ether, CAS: 16495-13-9; (R)-2-((Benzyloxy) methyl)oxirane, CAS: 14618-80-5) and glycidyl esters ((R)-Glycidyl butyrate, CAS: 60456-26-0; (S)-(+)-Glycidyl butyrate, CAS: 65031-96-1). Glycidol is also used as a diluent in some epoxy resins, as an additive for synthetic hydraulic fluids, and as a dye-leveling agent.
